Introduction: Cytomegalovirus (CMV) reactivation is a major complication among seropositive allogeneic hematopoietic cell transplantation (HCT) recipients; however, the incidence and outcomes of CMV reactivation in patients undergoing chimeric antigen receptor T (CAR T) cell therapy remain poorly understood.
Methods: Single-center retrospective study of 109 adult CMV seropositive patients who received CAR T cell therapy between February 2018 and February 2023. CMV viral load was monitored at the discretion of the treating physician. CMV outcomes were CMV reactivation (any viremia) and clinically significant CMV (viremia or tissue invasive disease requiring antiviral treatment). The study was approved by our institutional review board and conducted consistent with the principles in the Declaration of Helsinki. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism Software, version 10.0.0.
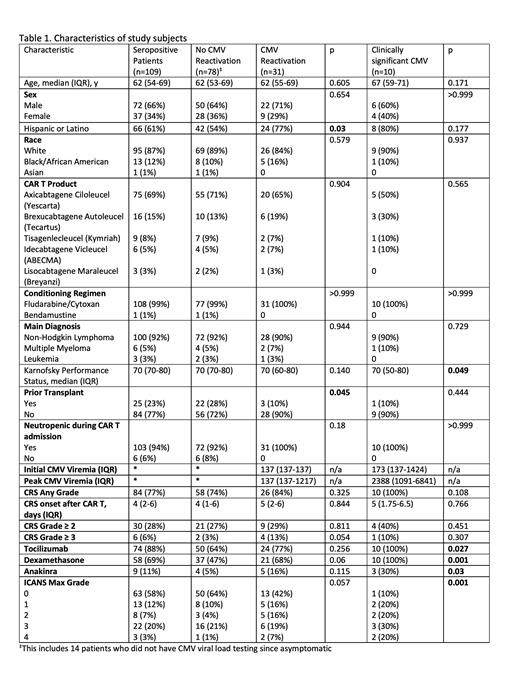
Results: During the study period, there were a total of 148 patients who received CAR T cell therapy. Patients who were CMV seronegative, had history of previous allogeneic HCT, or those who received letermovir prophylaxis were excluded. In total, 109 patients who were CMV seropositive met the inclusion criteria. The patient characteristics are presented in Table 1. Of the 109 patients, 69% received Axicabtagene Ciloleucel (Yescarta), 15% received Brexucabtagene Autoleucel (Tecartus), 8% received Tisagenlecleucel (Kymriah), 5% received Idecabtagene Vicleucel (ABECMA), and 3% received Lisocabtagene Maraleucel (Breyanzi). Eighty-four patients (77%) developed any grade of CRS with the median day of CRS onset being 4 days (IQR, 2 to 6). Forty-six patients (42%) developed any grade of ICANS. Among patients who developed CRS, 74 (88%) received tocilizumab, 58 (69%) received dexamethasone, and 9 (11%) received anakinra. For dexamethasone group, the median dosage was 103 mg (IQR, 45 to 160). In those receiving tocilizumab, the median number of doses was 2.5 (IQR, 1 to 4) and the dosage was standard 8 mg/kg. 31 patients (28%) had evidence of CMV reactivation (any viremia) and 10 patients (9%) had clinically significant CMV reactivation requiring treatment. The median time from CAR T cell infusion to CMV reactivation was 19 days (IQR, 9 to 31). Among those with clinically significant CMV reactivation, the median peak viremia was 2388 IU/mL; six of these patients (60%) developed CMV syndrome with none having tissue invasive disease; and 9 (90%) had documented clearance of the virus in response to antiviral therapy. In this group, there were 2 deaths but were not attributed to CMV reactivation. One was due to fungemia while the other patient's death was attributed to CAR T therapy related toxicities causing multiorgan failure. Among those who didn't reactivate CMV, 37 patients (47%) received dexamethasone, at a median dose of 90 mg (IQR, 25 to 145), whereas in the 10 patients with clinically significant CMV reactivation, 100% received dexamethasone (P=0.001) with a median dose of 130 mg (IQR, 74 to 263). Additionally, those who received tocilizumab and anakinra were also at a significantly higher risk for clinically significant CMV reactivation (p=0.027 and p=0.03, respectively).
Conclusions: CMV reactivation is a common complication of CAR T cell therapy in CMV seropositive individuals. Although confirmatory studies are needed, our data shows that administration of immunosuppression, in particular steroids, for management of CAR T cell toxicities is a major risk factor for CMV reactivation, and close monitoring for preemptive therapy or antiviral prophylaxis might be indicated in this setting.
Disclosures
Jimenez Jimenez:Abbvie: Research Funding. Beitinjaneh:Kite: Honoraria. Wang:Kite: Consultancy; Sanofi: Consultancy. Spiegel:ImmPact Bio: Consultancy; Kite Gilead: Consultancy.